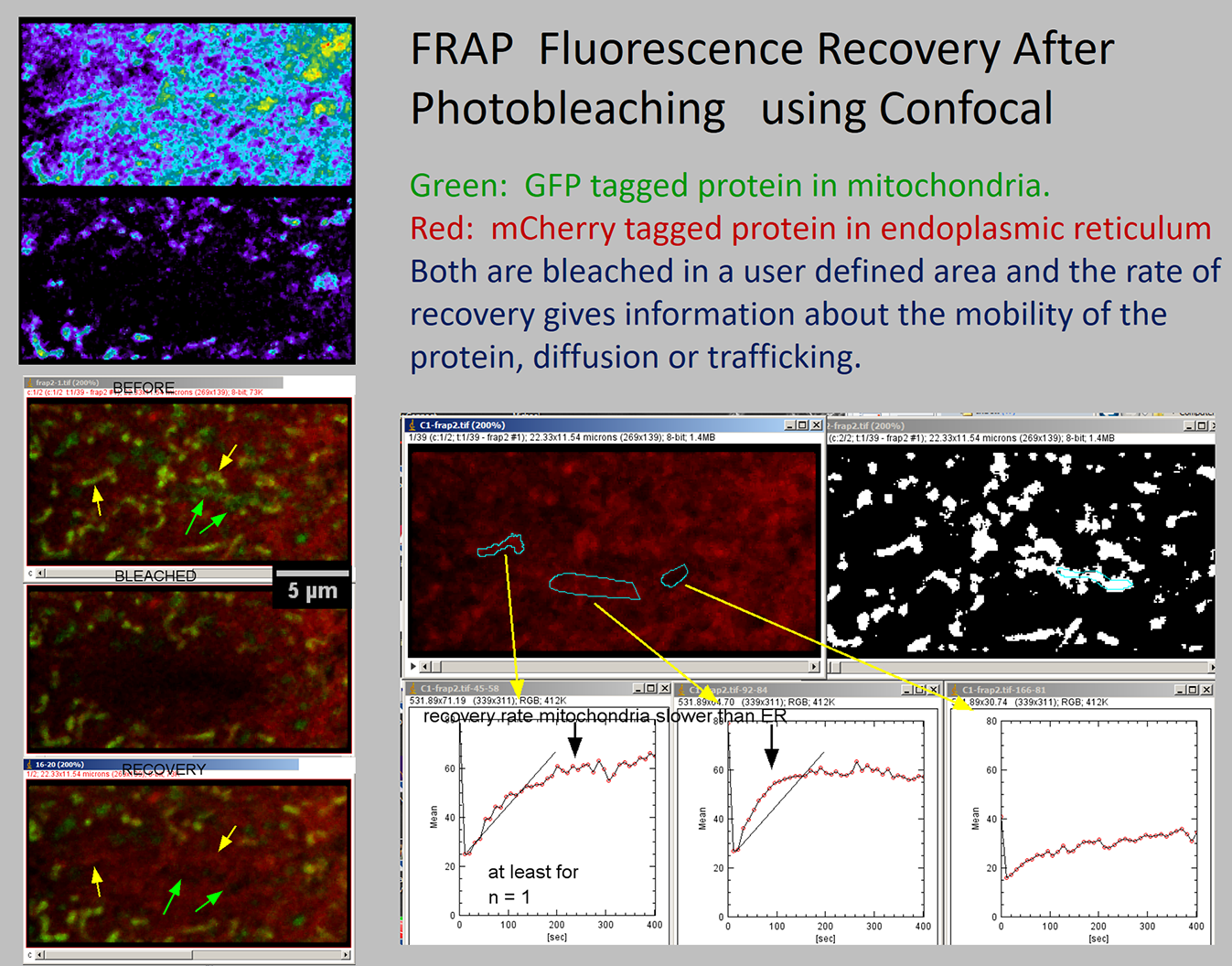
Fluorescence Recovery After Photobleaching (FRAP) is a technique where a small region within an area being imaged is bleached (without damaging the structure) and then imaged repeatedly to measure the rate of dark molecules moving out of the region and bright ones moving in to the region. As of January 2025, the Wikipedia page is a good resource for further explanation. More references are available at Nikon's microscopyu.
We have experience with FRAP in cells, tissues, lipid bilayers, and materials.
The process of FRAP is closely related to localized photoconversion or photodamage, the latter which can be at the molecular level (e.g. DNA) or at a more gross structural level (e.g. killing melanocytes in mouse skin or cutting skin of a zebrafish).
There are also variations such as continuously bleaching a region while imaging outside the region or localized photoconversion in a live animal and then hunting for cells that migrate to remote tissues (I'm still annoyed that I'm not an author on this paper or not named in the acknowledgements as I was the first person to get the Dendra to work in the tissue by using a Leica SP5).
An example of FRAP in characterization of protein hydrogels using a Leica Stellaris
From Fig 4 of Britton D, Christians LF, Liu C, Legocki J, Xiao Y, Meleties M, Yang L, Cammer M, Jia S, Zhang Z, Mahmoudinobar F, Kowalski Z, Renfrew PD, Bonneau R, Pochan DJ, Pak AJ, Montclare JK. Computational Prediction of Coiled-Coil Protein Gelation Dynamics and Structure. Biomacromolecules. 2024 Jan 8;25(1):258-271. doi: 10.1021/acs.biomac.3c00968. PMID: 38110299
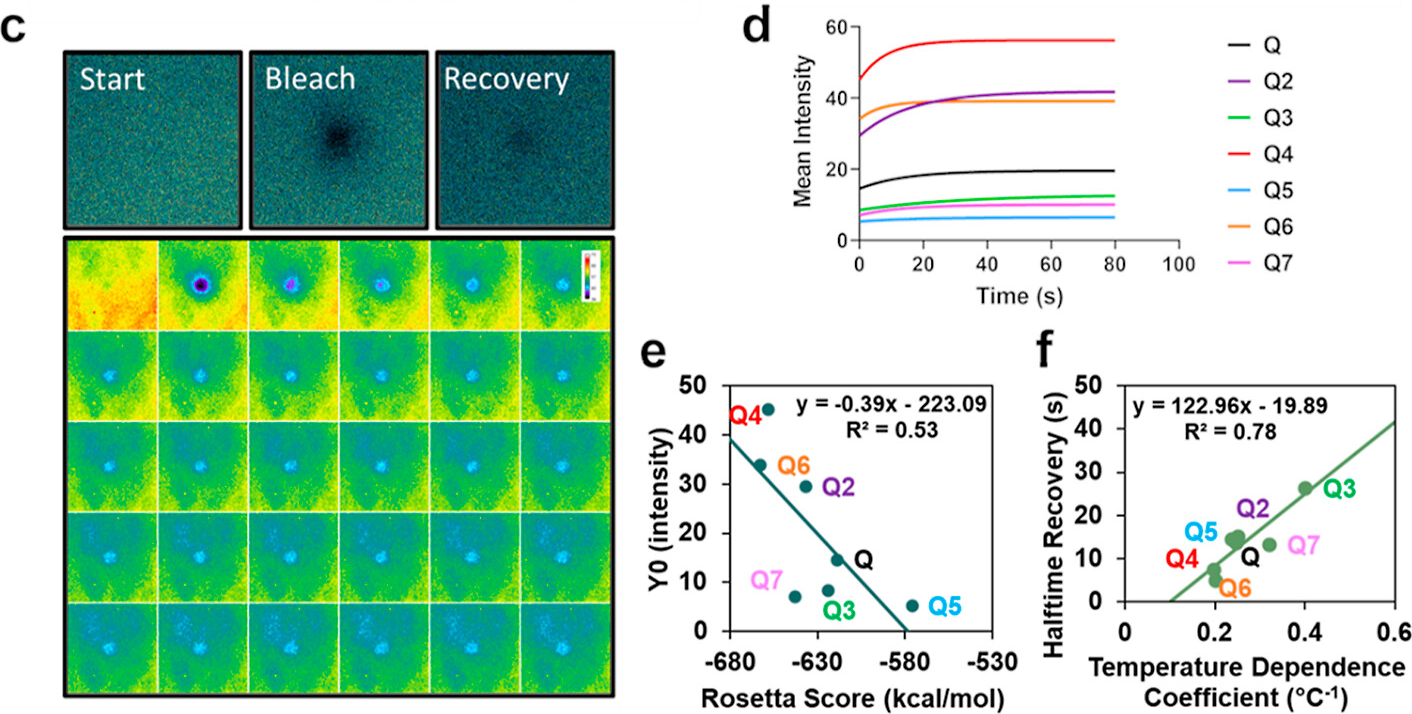
"(c) Representative FRAP imaging of the start (prebleach), bleach, and recovery (final postbleach) and corresponding montage showing the prebleach and the first 29 frames postbleach. (d) FRAP recovery curves were made by fitting to a one-phase association equation in Graphpad. (e) Linear correlation of Rosetta score to Y0 in one-phase association equation fit in Graphpad and (f) linear correlation of temperature dependence coefficient solved in respective phase diagrams to halftime recovery in one-phase association equation fit in Graphpad."
FRAP of lipid bilayers by widefield illumination. This method requires the user to very rapidly move parts on the microscope (done in 2011). A motorized scope with a software script could be more uniform.
Example of FRAP in neuron cell culture using Nikon A1 in 2017
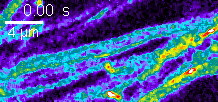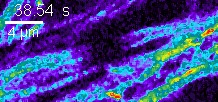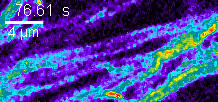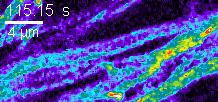
Zeiss 880 confocal FRAP in cell culture.
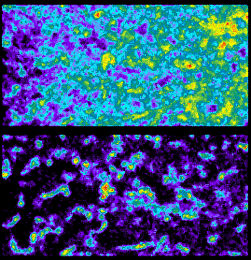
Pre-2001 BioRad confocal instructions for FRAP.
See:
Zeiss 880 and 710 and 700 confocals and Leica SP8 confocal
Spinning disk confocal with miniscanner
< Back



