Zeiss 880 Laser Scanning Confocal microscope with FCS, FLIM, and AiryScan
Location: SR C 17
Instructions startup/shutdown/general imaging: [Word doc] [PDF] How to do tiling.
Instructions AiryScan: [Webpage]
This microscope is most commonly used for standard fluorescence confocal. It also has other imaging modes, a few listed here:
- reflection, IRM
- transmitted light
- automated stage for tiling large areas or repeatably imaging multiple positions
- live and fixed samples
- FLIM (time domain, excitation with pulsed 440 nm laser)
- FCS and FCCS (continuous wavelength excitation)
A few of the specifications:
- Zeiss AxioObserver inverted stand
- environmental chamber for temperature and stage insert for CO2 control
- 10X air N.A. 0.3 -
For locating samples by eye, not recommended for confocal (we know you will use it anyhow...) - 20X air Plan-Apochromat N.A. 0.8 M27
For thinner samples, cell culture or thin cut tissue. - 25x/0.8 LD LCI Plan-Apochromat Imm Corr DIC M27 for oil, water, silicone oil or glycerine immersion (D=0-0.17mm) (WD=0.57mm at D=0.17mm)
Long working distance immersion lens great for cleared tissues. - 40X water N.A. 1.1 for standard imaging
- 40X water N.A. 1.2 for FCS
- 63X oil 1.40
- 100X oil N.A. 1.46 Use only for Airyscan and other specialty work.
NOT FOR ROUTINE CONFOCAL Instead, use the 63X with a zoom set or large pixel size image. - Excitation lasers: 405, 440 (pulsed for time domain FLIM), 458, 488, 514, 561, 594, 633 nm
- Detectors, path 1:
* PMT, 410 to 750 nm
* 32 channel spectral, 409 to 695 nm (example of true spectral detection here)
* redshifted GaAsP, up to 750 nm
* transmitted detector; use with Omega Optical 750SP filter in the condenser to block IR light from Definite Focus (see similar configuration on Zeiss 800 here). - Detectors, path 2:
* 2 GaAsP detectors ("BIG" detectors) with standard filter blocks for green/red and CFP/YFP - Detector, path 3:
* AiryScan (example with gamma tubulin)
(Note: In July 2025 a chatbot described Airyscan on this instrument this way: "It also improves signal-to-noise ratio and is excellent for live-cell imaging, tiling, and time-lapse experiments." To be clear, the version on this microscope cannot be used with tiling. Whether it is excellent for live imaging is arguable because switching between spectral channels can be exceedingly slow. AiryScan on this instrument is the first implementation.) - Automated Stage (tiling, repeated multiple positions)
- Definite Focus to lock focus for time lapse -- 750SP Omega Optical filter in condenser to prevent interference with transmitted light detector
- Windows 7 Zen Black
To adjust parfocaility using the touchpad:
Home > settings > focus > parfocality > adjustment
- How to open CZI files on your own computer:
1. https://www.zeiss.com/microscopy/us/products/microscope-software/zen-lite.html
2. ImageJ with BioFormats - Multifield timelapse setup instructions
- Explanation of noise, scan speed, and filtering for practical image quality.
- AiryScan example with gamma tubulin
- A method for labeling structure overlaps, here at high resolution using AiryScan data
- Example of FLIM
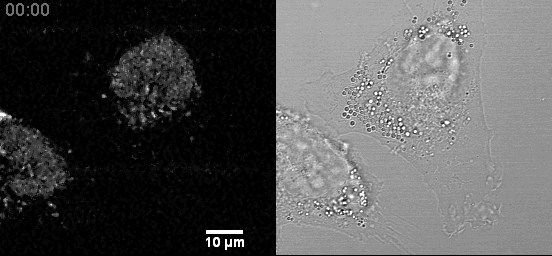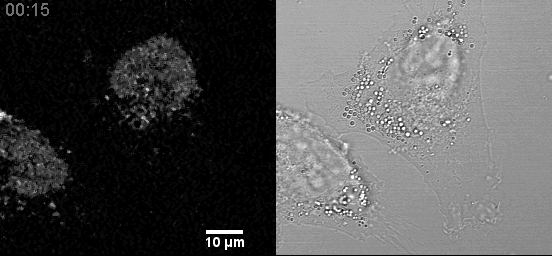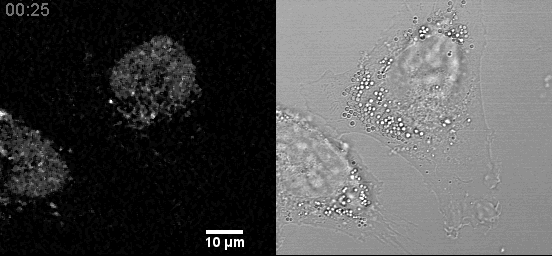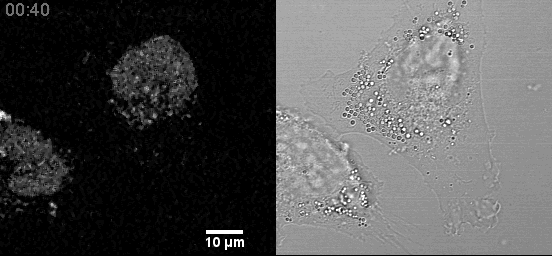
- Live cell mitochondria imaging with this microscope
Maintenance issues:
For service: Ser Nr 2802000293 ( or 2633000139 ??)
1-800-633-6610 1 1
MIC-SYSTEM Ser Nr 2802000293
Scanmodul 34Ch-G 24V= 1997-779
and
Rack LSM 880
Ser Nr 2817000414