
Zeiss AxioZoom microscope
Skirball 4th floor
To use microscope you must be trained by Michael Cammer or Yan Deng via the Microscopy Core.
** Signup to use microscope **

Starting up:
Shutdown DO IN CORRECT ORDER!!!
When using the computer, only store files on Drive D:
DO NOT STORE FILES ON DESKTOP or anywhere on Drive C: including "Pictures" folder.
If you want to see the Zeiss technical manual, it is here.
Protocol for 96 well plates here.
About the microscope
| Button | Useful for... |  |
| Dapi | Dapi, CFP (maybe), Pacific Blue Zeiss set 49: G365 - FT395 - BP445/50 |
|
| GFP | FITC, Alexa488, GFP, YFP BP470/40 - FT495 - BP525/50 |
|
| mRFP | RFP, mCherry, rhodamine, Cy3, Alexa568, Alexa594, propidium iodide BP572/25 - FT590 - BP 629/62 |
|
| BF | Transmitted light. (Also reflected light with an additional light source; sometimes there is one in the room or you could bring your own.) | |
| Shutter | Light on/off | |
| The filter cubes in this system are standard Zeiss and are simple to snap in and out. This means that if you have special spectral needs, we can swap out the blocks easily for your specific needs. We just need the block... | ||
| Crossed polarization methods are available and examples. |
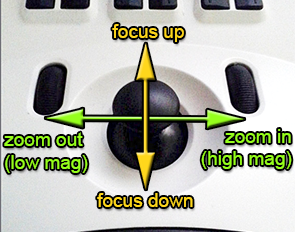 |
Push down to toggle to stage moving mode.
Push down to return to focus/zoom. |
When imaging fluorescence, to minimize reflections and maximize contrast, recommend these base settings.
You want to make sure the space below the sample is as blackand non-reflective as possible. (More details here if interested.)

For transmitted light imaging, recommend playing until you have the ideal image and then set the same every time for repeatability.
There are two lenses.
To change from one lens to the other, simply grap the lens and rotate to the next position.
The higher magnification lens focuses lower and closer to the sample. When switching to this lens, a message will be displayed on the touch pad. Please read it and follow thie instructions so that the lens does not crash into your sample or the stage.
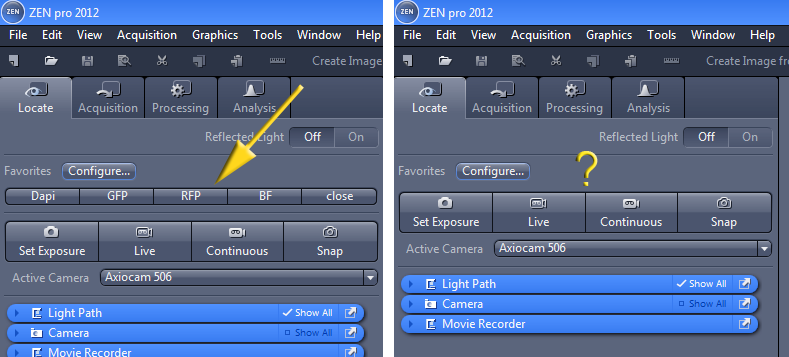
On software startup the defaults should have settings in the Locate tab for all fluorescent colors and brightfield.
| This is how the Locate window should look. The yellow arrow points to a row of buttons that control the microscope. | If the buttons are missing, then you need to reassign them using the Configure... button. We are trying to figure out why the buttons sometimes disappear so that we may stop this from happening. |
 |
|
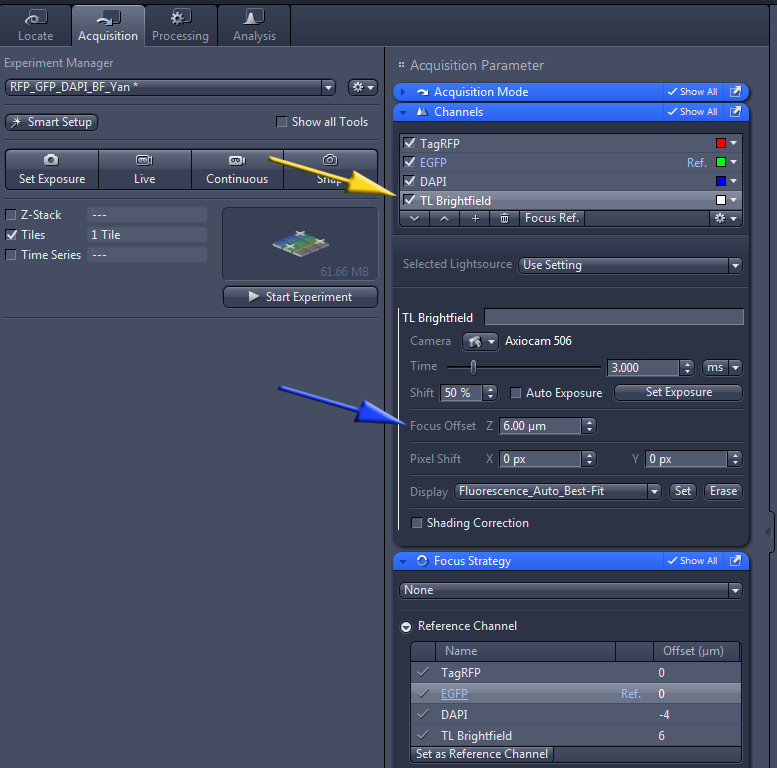
To preview and set up acquisition parameters for each channel, in the Acquisitiion tab click on the channel (yellow arrow below) and Show All in the blue bar above it. Note that you may set different focus settings for different channels.
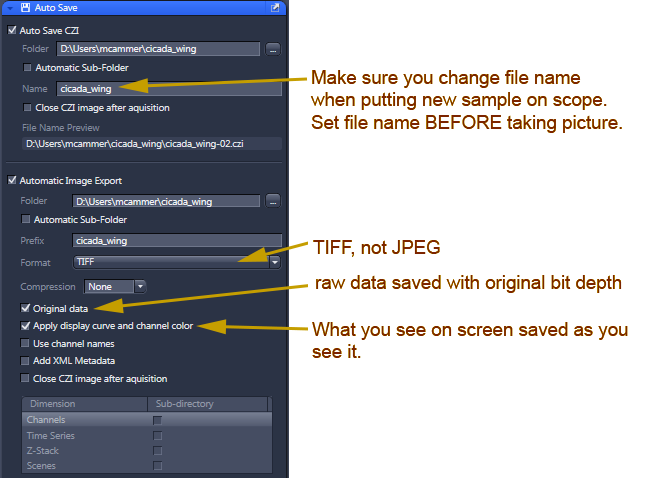
Software installed May 2016 should have this Auto Save menu option for saving files:
Crossed polarization methods are available and examples.
comments, questions, suggestions for this web page: Michael.Cammer@med.nyu.edu or mcammer@gmail.com