Images collected with Zeiss 880 confocal.
Permeabilization
The first set of pictures in this web page shows what happens when fixed cells are not permabilzed and an attempt is made to label a specific molecule inside the cell.
In 9th grade NYS Regents biology we learned that mammalian cells had lipid membranes which made them impermeable to the environment around them. Seems this continues to hold true today.
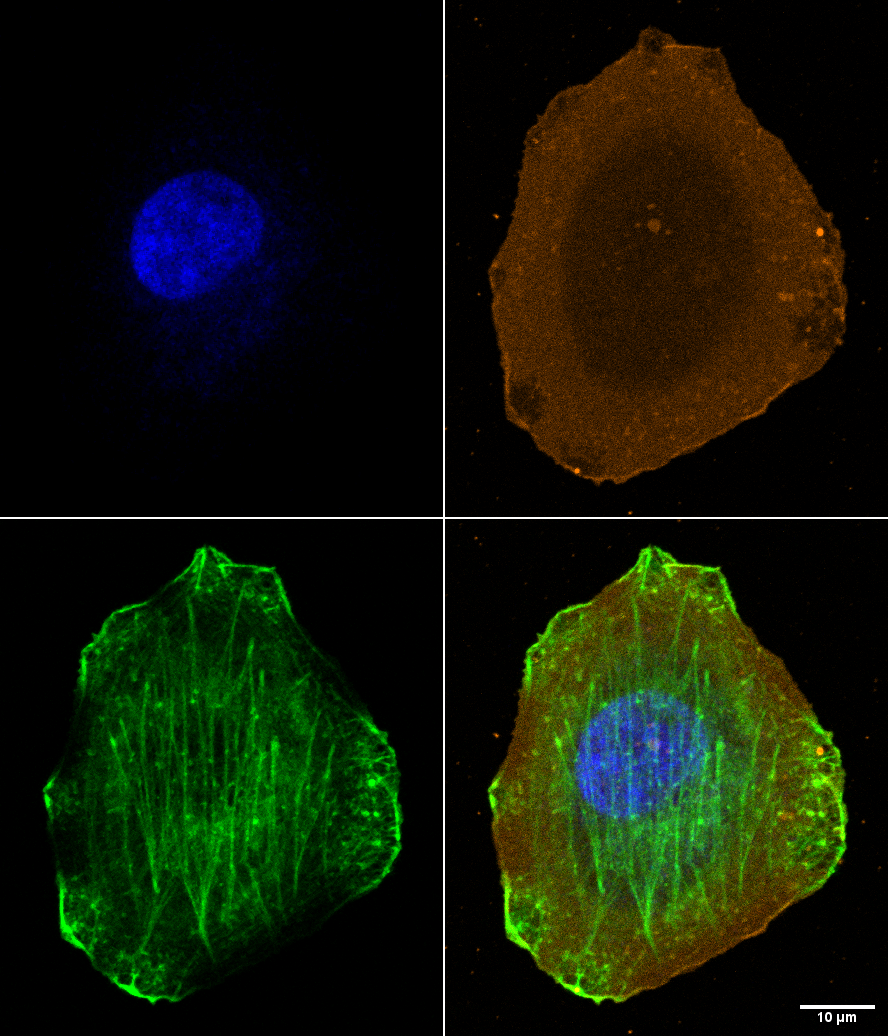
The image below shows a mammalian vero cell in culture on a #1.5 coverslip that was bathed in a red dye (attached to a lectin maybe?) that bound to proteins exposed onthe outside of the cell membrane and then was fixed with fresh 2% PFA. While imaging the red probe (orange), DNA labeled with dapi (blue), and transmitted light (gray) we decided to try adding Alexa 488 phalloidin to light up the f-actin (2 ul into 1 ml). The green image shows that a lot of the actin gets labeled, but there are unlabeled patches. Also, the brightest regions at the periphery appear to correspond to unlabled patches in the orange or, more likely than unlabeled, are regions where the membrane was damaged thus allowing entry of the AF488-phalloidin.
 |
 Images collected with Zeiss 880 confocal. |
We are used to seeing actin filament labeling as uniform around the cell as shown in the following picture (different but morphologically similar cell type):
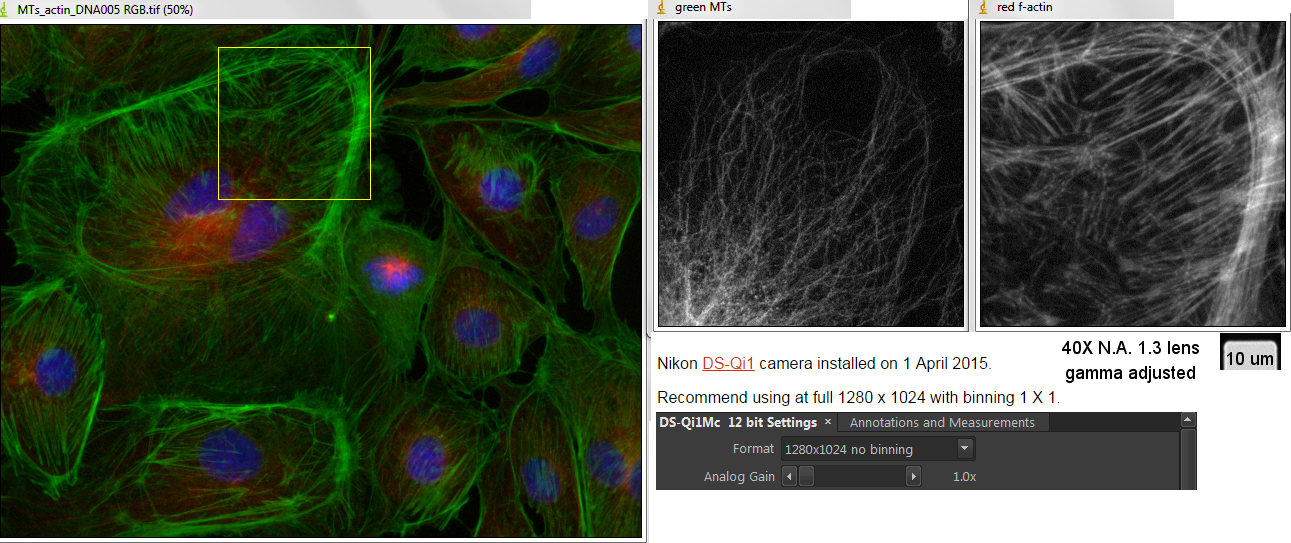
The difference is that a surfactant (Triton X-100) was used to remove the lipids which allowed the AF488-phalloidin to access the actin.
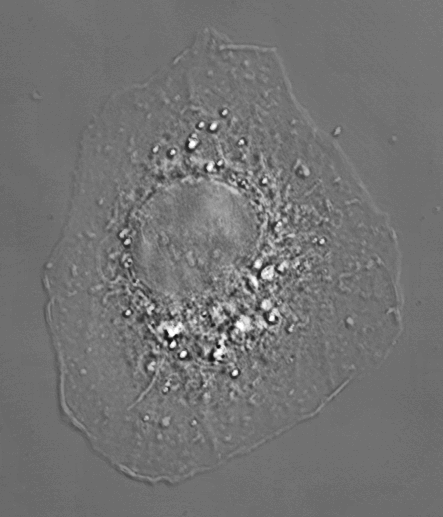
On the other hand, because extraction does wash out lipids, it may create other artefacts. Here is an example of a cell imaged by refelction live, fixed (although without special buffering), and Triton extracted which show changes in morphology at each step.
On the other hand, maybe the bald spots really don't have f-actin in them. We didn't then add Triton to check. But according to images on the internet, such as this one, we think we're right.