Quick tissue screening or high quality final data with super simple preparation.
Simple Prep.
Fluorescent protein expressing whole organ dissected and dropped into a tube of 2% to 4% PFA in buffer. An hour later (or next day) wash the tissue in buffer, immobilize in a dish, and pour PBS over it. (If don't want to submerge, put Genteal on it.)

To get a result like this (not the same tissue as shown above):
 |
 |
|---|
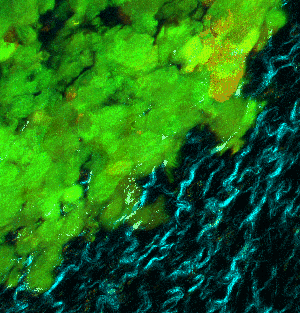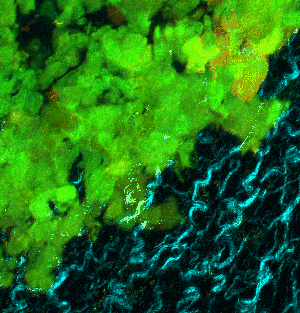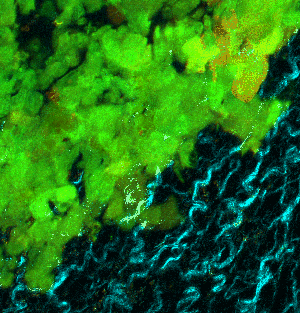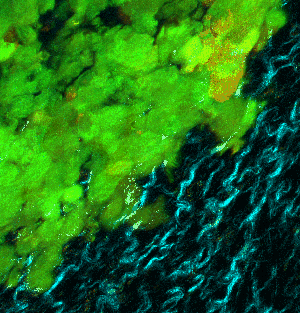
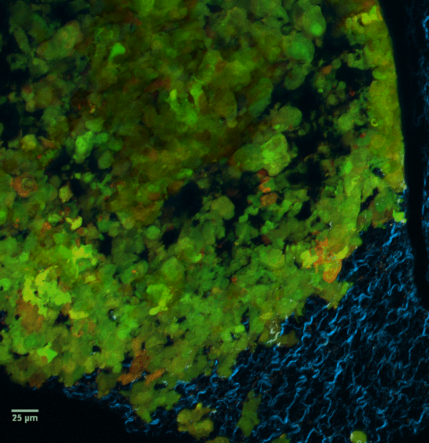
At left GIF animation of subset of Z series cropped from 1024 x 1024 pixels full resolution with 1X zoom scanned in galvo mode. At right, maximum pixel projection through subset of the Z series spatially scaled in XY at 50%.
This allows you to look at may tissues quickly, not just your tissue of interest. You may find things in tissue outside your speacialty that informs your research in unexpected ways. This method facilitates encourages looking more and faster.
Other variations:
Inject fluorescent probe to see vasculature.
Incubate in 0.1% Triton overnight mixed with fluorescently labeled phalloidin, dapi, or other small molucules with fluorescent probes.
Click on the next two images for full size versions:
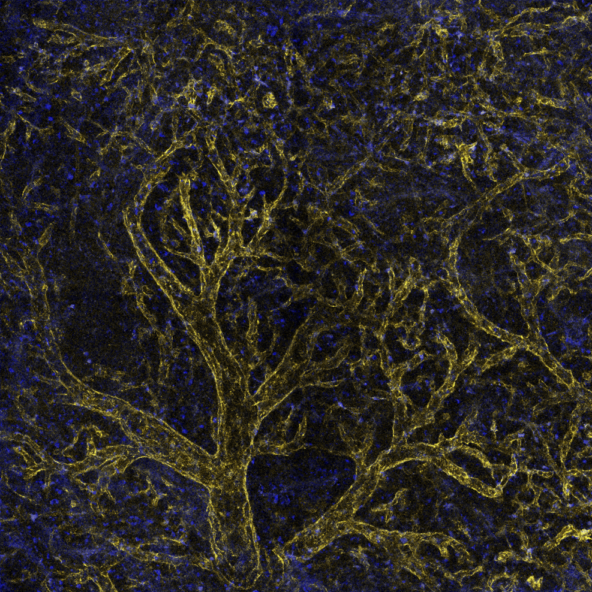 |
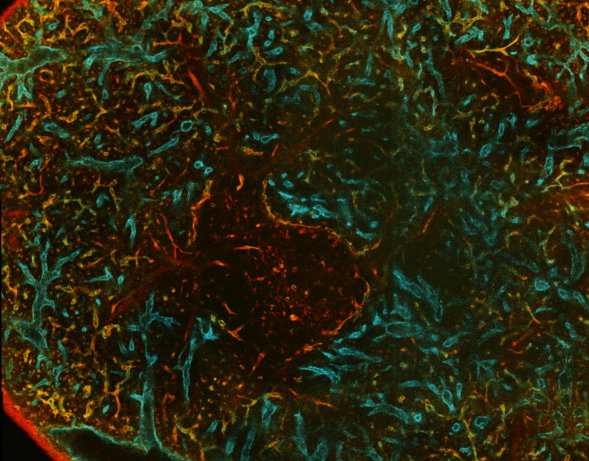 |
3D examples of other spleens, click images below:
 |
 |
 |
 |
The following nail & skin images were scanned on Zeiss 710 a few years previous.
Click here for a 3D movie collected by multiphoton.
Mouse footpad same labels as nail above.