Polarized light microscopy with AxioObserver.
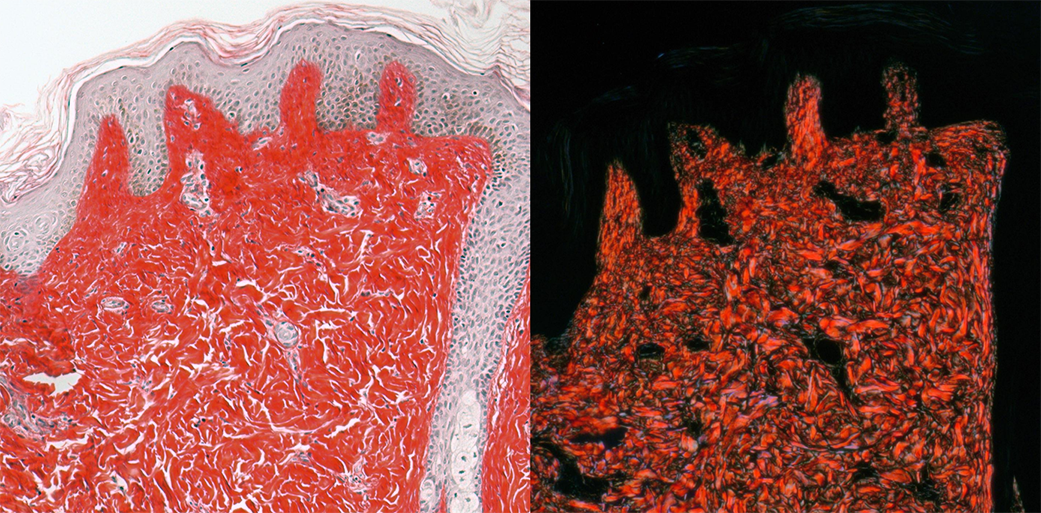
LEFT: Brightfield image of chromogranic stained skin (prepared by Histology Core).
RIGHT: Crossed polarization image of same sample. Note that only the collagen depolarizes the light and that direction of collagen can be discerned in the images. (This can also be done with unstained tissue and with fluorescently stained tissue.)

Set up two channels using the color camera and the light set to 3200K for best wite balance. May need to have camera gain at 1.
These methods need to be checked, but this is what I recall doing:
Channel 1, polarized light.
The DIC prism needs to be set in the turret that contains the fluorescent filter cubes.
The brightfield setting needs to be in the light path at the top.
Rotate polarizer in condenser 90 degrees??
Channel 2, standard brightfield:
Same as above except remove the DIC prism in the bottom turret.
Each channel needs to be set to no LUT.
To keep halogen bulb happy and for best illumination, in light path at left, set it to stay on all the time.
Could also set shutter to never close for faster imaging.
In ZEN, images need to be viewed in split mode.
If new version of Zen allows this, autosave may be set to automatically save each channel separately.
Crossed polarization can be done at lower magnification with the AxioZoom.
Example:
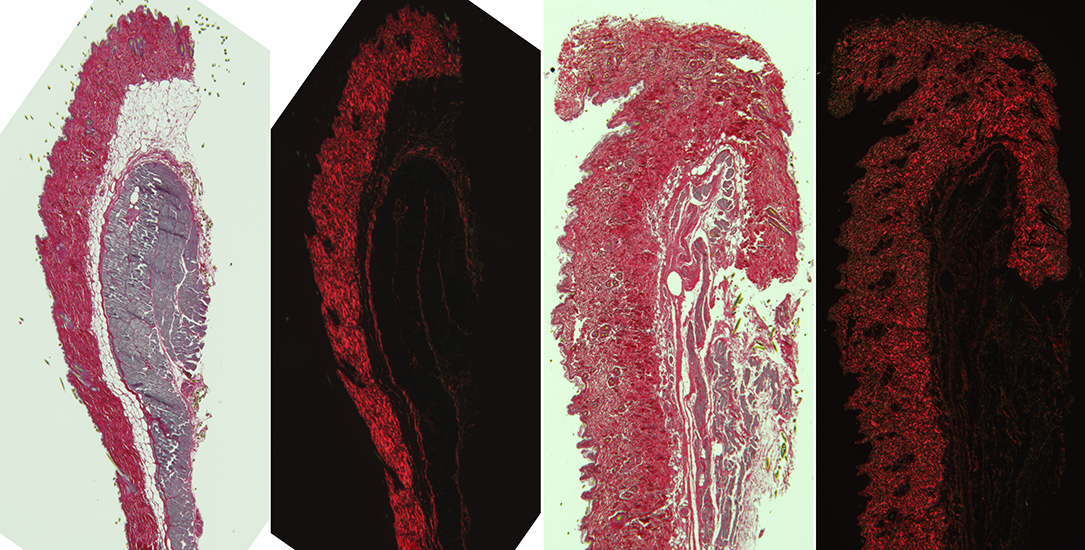
Other methods for imaging collagen:
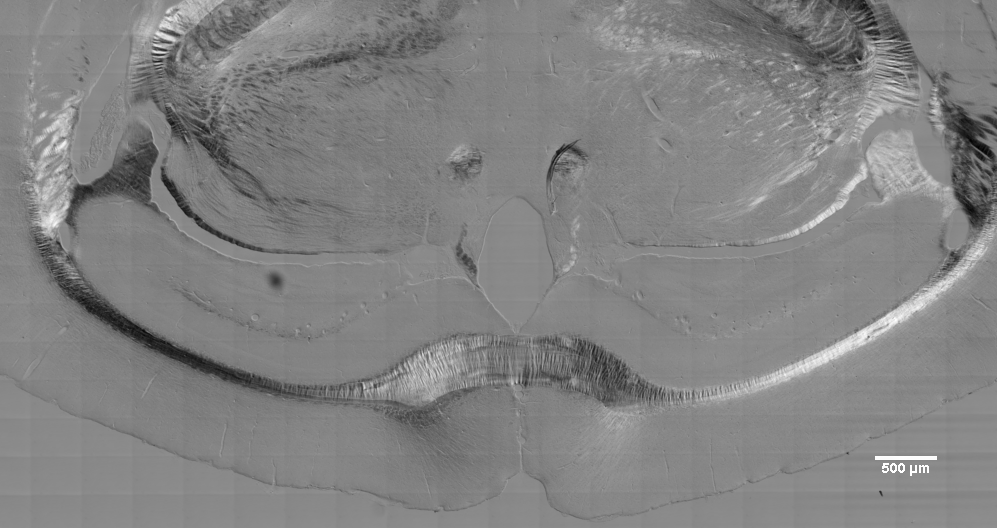
Another example of polarized light. The 20X air lens was used with DICII optics in the lightpath. Tiling was done with 1x1 binning and the images drastically rescaled to show here.
The point is that the dark/light effects of polarized light are laterally symmetric, light to the left of the midline and dark to the right and vice versa.
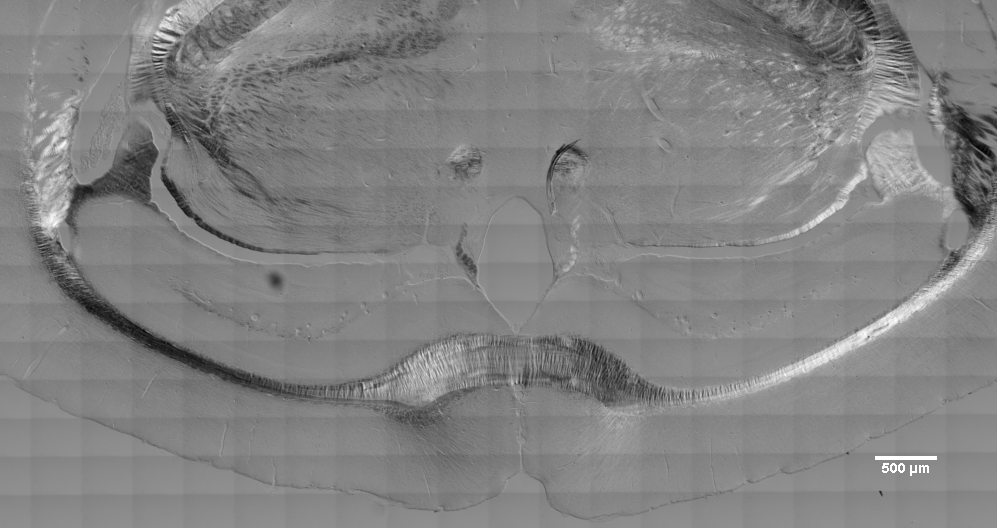
Below fft filtered to get rid of the tiling grid (unfortunately, scalebar stamped on image so caused ringing in lower right corner).